Dosing and administration

For treatment of GPP flares1
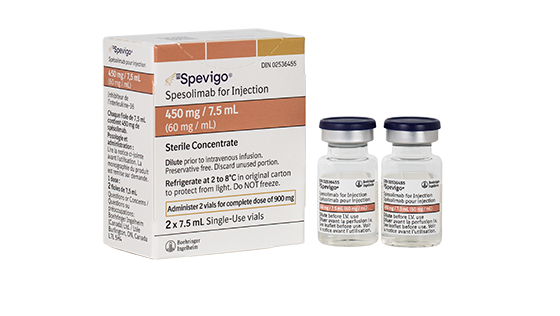
The recommended dose SPEVIGO® solution for infusion:
one 900 mg IV infusion
(2 x 450 mg/7.5 mL vials)
If flare symptoms persist, an additional 900 mg dose (2 x 450 mg/7.5 mL vials) may be administered 1 week after the initial dose.
Treatment with SPEVIGO® for intravenous infusion should be initiated by physicians experienced in the management of patients with inflammatory skin diseases.
SPEVIGO® solution for infusion is intended for IV use for GPP flare treatment only.
For prevention of GPP flares1
The recommended dose of subcutaneous SPEVIGO®:
one 600 mg SC loading dose
(4 x 150 mg injections)300 mg SC q4w thereafter
(2 x 150 mg injections)
If a patient experiences a GPP flare while receiving subcutaneous SPEVIGO®, the flare should be treated with intravenous SPEVIGO®.
Four weeks after treatment with intravenous SPEVIGO®, subcutaneous SPEVIGO® can be initiated or reinitiated at a dose of 300 mg (2 x 150 mg injections) administered q4w. An SC loading dose is not required.
SPEVIGO® solution for injection in a pre-filled syringe is intended for SC use for GPP flare prevention only.

SPEVIGO® treatment can be initiated with SPEVIGO® as an SC injection to prevent GPP flares or SPEVIGO® IV to treat a GPP flare.
Please see the Product Monograph for complete dosing and administration details.
IV=intravenous; q4w=every 4 weeks; SC=subcutaneous.


